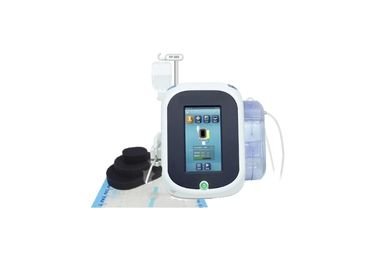
Wound vac canister, Dressing Kits
wound vac canister is disposable and an accessory of negative pressure wound therapy products. Consumable, for single patient use, be replaced regularly.
Multiple Capacity Options: 300 ml,500ml ,and 1000ml
Wound Vac Therapy System
- Certifications
- Manufacturing
Consumable of NPWT, wound vac canister
- Wound pressure detection connector
- Suction tube connector
- Overflow protector
- Snap-fit joint

Canister overflow prevention ,Highly absorbent gel, capable of absorbing excess waste liquid in canister
Anti-sloshing canister,The maze design inside prevents the waste from flowing into the main unit after the machine has been dropped
How to connect the suction tube to the wound vac canister?
Connect the two joints of the suction tube respectively to the wound pressure detection connector and Suction tube connector on the wound vac canister.

Note:
When the overflow protector is in contact with water, the device will stop working. Replace the wound vac canister with a new one, and tap “Start”, the device will resume working. The overflow protector is inside the canister and requires no installation/debugging by the user; after pipelining, it can function normally when operated as per the User Manual.
It is unnecessary to empty the wound vac canister; after a single use, press the unlocking button to remove the canister from the main unit, and dispose of it according
to the hospital’s rules on the disposal of medical waste.
NPWT, stands for Negative Pressure Wound Therapy, referring to wound dressing systems that continuously or intermittently apply negative pressure to the surface of a wound. NPWT has become a popular treatment modality for the management of many acute and chronic wounds.
Only doctors or trained nurses are recommended to operate the products or change dressings.
We have obtained the CE certificate, Chinese Free Sales certificate, ISO13485 certificate, and so on, for the device.
We understand that different countries have different regulations for importing medical devices. So yes, we could provide the required documents to our customers. Please contact our team for details.